FDA Approves Pfizer Vaccine
Monday, August 23rd, 2021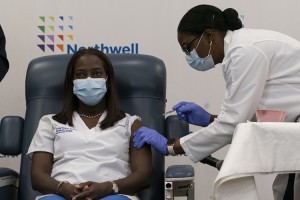
Sandra Lindsay, left, an African American nurse, is injected with the COVID-19 vaccine developed by Pfizer on Dec. 14, 2020, in the Queens borough (section) of New York City. The rollout of the vaccine, the first to be given emergency authorization by the Food and Drug Administration, began the biggest vaccination effort in U.S. history.
Credit: © Mark Lennihan, Getty Images
On Monday, Aug. 23, 2021, the United States Food and Drug Administration (FDA) fully approved the two-dose Pfizer vaccine for COVID-19. The FDA has granted full approval of the vaccine for those aged 16 years and older. Full approval of a vaccine will make it easier for public and private organizations to require vaccinations. This includes hospitals, active-duty military, and schools.
The coronavirus disease COVID-19 has killed more than 4 million people and infected more than 200 million people around the world. The first countries with access to a vaccine began vaccinating their citizens in December 2020. Almost 5 billion doses of a vaccine preventing COVID-19 have been administered to people around the world.
In late November 2020, the companies Pfizer and Moderna each applied for emergency approval from the FDA for their COVID-19 vaccines. The two companies are among dozens of drugmakers that worked tirelessly to develop a vaccine against the deadly virus. Because the vaccine was authorized for emergency use after a clinical trial of 40,000 people, many citizens were hesitant to receive the vaccine. Full approval of the vaccine may assure some of those yet to get the shot of the vaccine’s safety.
Pfizer and Moderna began clinical trials in July. During these trials, participants were given either the vaccine or a placebo. A placebo is a substance that contains no active ingredient. Comparing infection rates in subjects who received the placebo with those among subjects who got the vaccine can help determine if the vaccine is effective. In the Pfizer and Moderna clinical trials, half the participants were given a placebo of saltwater, and half were given the vaccine. The researchers then waited to see who might get sick. The results were very promising—both vaccines were about 95 percent effective in preventing COVID-19. By contrast, commonly administered influenza vaccines (known as flu shots) are 40 percent to 60 percent effective.