“V-Day” Arrives in the U.S.
Tuesday, December 15th, 2020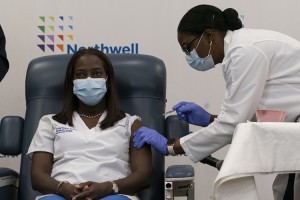
Sandra Lindsay, a Black nurse, is injected with the COVID-19 vaccine developed by Pfizer on Dec. 14, 2020, in the Queens borough (section) of New York City. The rollout of the vaccine, the first to be given emergency authorization by the Food and Drug Administration, begins the largest vaccination effort in U.S. history.
Credit: © Mark Lennihan, Getty Images
The long-awaited V-Day, short for Vaccine Day, arrived in the United States on Monday, December 14, with the beginning of widespread vaccination against the coronavirus disease COVID-19. Vaccination involves the administration of special medicines called vaccines, which can help make a person immune to a particular disease. Sandra Lindsay—a nurse at a hospital in the Queens borough (section) of New York City—became the first person in the United States to receive the authorized COVID-19 vaccination. COVID-19 has killed more than 1 million people and infected more than 60 million people around the world. The United States Food and Drug Administration (FDA) granted an emergency use authorization for the vaccine, by the pharmaceutical company Pfizer, on December 11. V-Day provided a moment of hope against the COVID-19 pandemic (worldwide outbreak of disease) even as the U.S. death toll topped 300,000.
The fact that Lindsay—a Black health care worker—was first in line to receive the vaccine is significant. In the United States, COVID-19 has disproportionally affected Black Americans, and medical workers have been on the front line of the fight against the disease. Lindsay said it was important for her to take the vaccine, in part because of the history of unequal and racist treatment of minorities in the medical system. In particular, she mentioned the Tuskegee Syphilis Study, a notorious medical experiment involving Black Americans. Beginning in 1932, medical workers conducted blood tests among 4,000 Black men in Tuskegee, Alabama, and selected for the study about 400 who were found to be infected with the sexually transmitted disease syphilis. The participants in the study were not informed that they were infected with syphilis or told about the expected outcomes of the experiment. Lindsay hoped to inspire Black people and other minorities who may be skeptical about the vaccine. After receiving the first of two doses, she said, “It feels surreal. It is a huge sense of relief for me, and hope.”
The first doses of the Pfizer vaccine to be given to Americans were shipped on Sunday, December 13. The United States is not the first country to approve the vaccine. On December 8, Margaret Keenan of the United Kingdom became the first person in the world to receive the authorized vaccine. Canada has also approved the vaccine, administering its first dose the same day as the United States.
In many cases, administration of a COVID-19 vaccine will be voluntary. But, it will be a while until everybody who wants a vaccine can get one. In the United States, the first doses will be given to health care workers. Frontline workers (workers likely to encounter the disease) and people who are vulnerable to the virus, including the elderly and people with such risk factors as obesity or diabetes will be next. Some of these people may receive the vaccine by the end of 2020. But, most people will have to wait until the spring of 2021.
Most vaccines are administered into the body by injection. A vaccine contains substances that stimulate the body’s immune system to produce molecules called antibodies. The immune system uses antibodies to fight against germs that enter the body. Antibodies produced in response to a vaccine can protect a person who is exposed to the actual disease-causing organism. The process of protecting the body in this way is called immunization. Vaccines have been successful in fighting many other diseases, including chickenpox, meningitis, and yellow fever.
Pfizer and Moderna began clinical trials in July. During these trials, participants were given either the vaccine or a placebo. A placebo is a substance that contains no active ingredient. Comparing infection rates in subjects who received the placebo with those among subjects who got the vaccine can help determine if the vaccine is effective. In the Pfizer and Moderna clinical trials, half the participants were given a placebo of salt water, and half were given the vaccine. The researchers then waited to see who might get sick. The results were very promising—both vaccines were about 95 percent effective in preventing COVID-19. By contrast, commonly administered influenza vaccines (known as flu shots) are 40 percent to 60 percent effective.
In late November, the companies Pfizer and Moderna each applied for emergency approval from the FDA for their COVID-19 vaccines. The two companies are among dozens of drugmakers that have worked tirelessly to develop a vaccine against the deadly virus.


