Periodic Table 150
Wednesday, March 6th, 2019March 6, 2019
On March 6, 1869, 150 years ago today, the Russian chemist Dmitri Mendeleev presented his version of the periodic table to the Russian Chemical Society in Moscow. The periodic table arranges the known chemical elements into periods (rows) and groups (columns) based on their characteristics. A chemical element is a substance that contains only one kind of atom. Attempts to create a standard table of the chemical elements predated Mendeleev, but his version was quickly accepted and is still in use—with only slight modification—today.
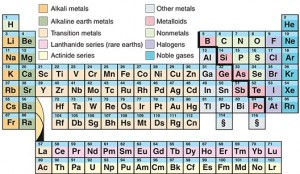
The modern periodic table is similar to the table developed by the Russian chemist Dmitri Mendeleev in 1869. Credit: WORLD BOOK
The periodic table ranks as one of the most useful tools in chemistry and perhaps in all of science. No other scientific field has such a simple but descriptive means of representing its fundamental units. Elements in the same column share certain chemical properties (characteristics). These properties repeat row by row in a regular or periodic fashion, giving the table its name.

The Russian chemist Dmitri Mendeleev developed the modern periodic table of elements. Credit: Public Domain
The French chemist Antoine Lavoisier first established the modern concept of the chemical element in the late 1700’s. As more and more elements were discovered, chemists noticed that the elements show certain chemical similarities and trends in their properties. In a paper published in 1829, the German chemist Johann Döbereiner grouped some elements into triads (groups of three) based on the similarities in their chemical properties. After the discovery of more elements, the English chemist John Newlands noted around 1865 that if the elements are arranged according to their atomic masses (amounts of matter), their chemical properties repeat after eight elements.

Click to view larger image
Mendeleev’s Russian language periodic table was published 150 years ago in 1869. Credit: © The Print Collector/Alamy Images
Two qualities of Mendeleev’s 1869 table made it superior to previous attempts. First, Mendeleev did not organize the elements strictly by mass. Instead, he switched the order of some elements on the basis of their chemical properties. Second, he deliberately left gaps in his table where no known elements seemed to fit. Mendeleev correctly predicted that these gaps represented elements that had not yet been discovered.
Around the same time as Mendeleev, the German chemist Julius Lothar Meyer independently developed a similar periodic table. However, his table was not published until 1870.


