Periodic Table Scores Its Seventh Complete Row
January 5, 2016
The year 2016 heralded the addition of four new chemical elements—the building blocks of matter—to the periodic table. The new elements, which have yet to be officially named, are numbers 113, 115, 117, and 118 on the table. The International Union of Pure and Applied Chemistry recognized the new elements on Dec. 30, 2015. Teams of scientists from America, Japan, and Russia discovered them.
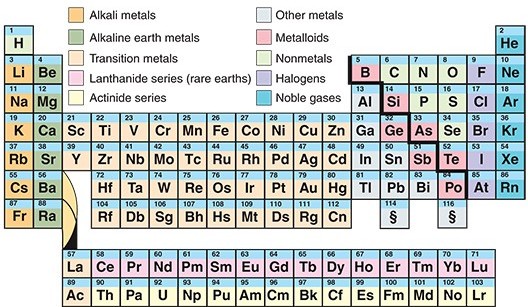
The periodic table is one of the most useful tools in science. It organizes the chemical elements into rows and columns, which show how the elements are related and what properties they share. For example, elements in the left and center of the table are metallic. Elements in the far right column, called the noble gases, do not normally react with other elements. The discovery of elements 113, 115, 117, and 118 fills all the remaining gaps in the periodic table’s seventh row.
Each chemical element is a kind of atom. The core of an atom, called a nucleus, contains even smaller particles called protons. An atom’s proton count determines its number on the periodic table. The lightest element, hydrogen, has just one proton, so it is the first chemical element on the table. Helium—element #2—has two protons. Carbon has six protons, oxygen has eight, and iron has 26. The more protons an element has, the heavier it is.
Extremely heavy elements are unstable, tending to fall apart like towers of loose blocks. Elements 113, 115, 117, and 118 are so heavy and unstable that they do not even exist in nature. Scientists created them in the lab by smashing together the nuclei of lighter atoms. They then observed the resulting new atoms—easier said than done, since the heavy atoms disintegrate into lighter atoms and particles in just a fraction of a second.
Other World Book articles: